ABOUT ME
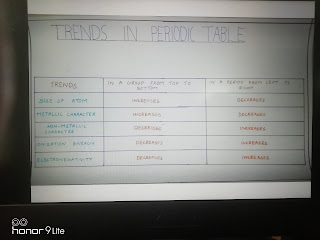 |
| TABLE CHART |
ABOUT ME
 |
| TABLE CHART |
NSS TRAINING COLLEGE OTTAPALAM -
ICT WORKSHOP DIGITAL TEXT
Name of teacher: Silfa Anna Joy
Department : Physical Science
Class : 8 th
Subject : Chemistry
Unit : Chemical changes
Objectives:
● Identify different changes happening in the surroundings.
● Discuss the difference between physical and chemical changes.
● Appreciate the importance of knowledge on physical and chemical change in our life through citing its application in our community.
● Identify examples of physical and chemical changes.
INTRODUCTION
Every day you come across so many changes. Changes can be of different types:-desirable and undesirable changes, slow and fast changes, temporary and permanent changes, etc...In this chapter, we shall study about nature of these changes.
Content
Broadly changes are classified into two types: Physical changes and chemical changes.
PHYSICAL CHANGES
Take plain white paper and cut it into square pieces. Here the shape of the paper, that is only its physical properties changed. But is there any change in the chemical properties of this paper? No, there is no change in its chemical property.
Thus the change in which a substance undergoes a change in its physical properties like size, shape, color, and state of a substance is called a physical change. A physical change is a temporary change and is reversible.No new substance is formed in this type of change.
Examples of physical change include melting an ice cube, crushing a pan, boiling water, water becoming water vapor on heating, breaking a bottle, chopping vegetables, mixing sand and salt, making sugar crystals,dissolving sugar in water, etc…
CHEMICAL CHANGES
Take plain white paper and burn it. what do you see? Is a new substance formed here? Yes, when a paper is burned ashes are formed and we cannot obtain that plain white paper back from these ashes. so it's a permanent irreversible change.
A chemical change occurs when two or more chemical substance reacts to form a new product that has an entirely different set of properties. It is a change at the molecular level of matter. Chemical bonds between atoms break and then form to connect different atoms.
Examples of chemical changes include souring milk, digesting food, cooking an egg, baking a cake, rusting of iron, burning of firewood, etc…
ABOUT ME Myself Silfa Anna Joy. I completed my schooling at Mount Carmel GHSS, Kottayam and I graduated with BSc i...
